Deep Removal of Fluoride by Highly Activated Magnesium Oxide
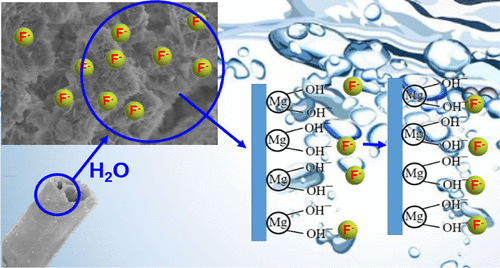
This study successfully prepared active magnesium oxide (MgO) for efficient removal of low-concentration fluoride ions using basic magnesium carbonate as raw material through calcination. The effect of calcination temperature on material properties was systematically investigated. Experimental results indicate that MgO calcined at 500°C exhibits optimal adsorption performance, featuring a large specific surface area, abundant active sites, the highest adsorption capacity for fluoride ions, and the fastest adsorption kinetic rate.
Under optimal adsorption conditions (initial fluoride concentration of 20 mg/L across a broad pH range), this material achieved a fluoride removal rate of 98.35% with a maximum adsorption capacity of 19.3 mg·g⁻¹, significantly outperforming most reported metal oxide adsorbents. Coexisting ion interference experiments revealed that Cl⁻, CO₃²⁻, and HCO₃⁻ exerted minimal effects on the adsorption process, whereas SO₄²⁻ and NO₃⁻ exhibited pronounced competitive adsorption effects, likely due to their competition with fluoride ions for coordination at MgO surface active sites.
Adsorption mechanism studies indicate the process follows a pseudo-second-order kinetic model and Langmuir isotherm model, confirming monolayer chemical adsorption. The adsorption process comprises two stages: an initial rapid adsorption phase governed by surface diffusion, and a subsequent slow adsorption phase controlled by intra-particle diffusion. Characterization techniques including FTIR and XPS further elucidated the adsorption mechanism: F⁻ ions form O–H···F hydrogen bonds with MgO surface hydroxyl groups, while some F⁻ ions may undergo ion exchange with Mg²⁺ or form surface complexes, leading to effective fluoride ion immobilization.
In summary, the developed active MgO material offers advantages including simple preparation, high adsorption efficiency, and a broad pH applicability range, presenting a promising adsorbent option for advanced treatment of drinking water and fluoride-containing wastewater. Future research may focus on the material's recyclability, adsorption selectivity under complex real-water matrices, and the development of compact water purification devices based on this material. This study successfully prepared active magnesium oxide (MgO) for efficient removal of low-concentration fluoride ions using basic magnesium carbonate as raw material through calcination. The influence of calcination temperature on material performance was systematically investigated. Experimental results indicate that MgO calcined at 500°C exhibits optimal adsorption properties, featuring a large specific surface area, abundant active sites, the highest adsorption capacity for fluoride ions, and the fastest adsorption kinetic rate.
Under optimal adsorption conditions (initial fluoride concentration of 20 mg/L across a broad pH range), this material achieved a fluoride removal rate of 98.35% with a maximum adsorption capacity of 19.3 mg·g⁻¹, significantly outperforming most reported metal oxide adsorbents. Coexisting ion interference experiments revealed that Cl⁻, CO₃²⁻, and HCO₃⁻ exerted minimal effects on the adsorption process, whereas SO₄²⁻ and NO₃⁻ exhibited pronounced competitive adsorption effects, likely due to their competition with fluoride ions for coordination at MgO surface active sites.
Adsorption mechanism studies indicate the process follows a pseudo-second-order kinetic model and Langmuir isotherm model, confirming monolayer chemical adsorption. The adsorption process comprises two stages: an initial rapid adsorption phase governed by surface diffusion and a subsequent slow adsorption phase controlled by intra-particle diffusion. Characterization techniques including FTIR and XPS further elucidated the adsorption mechanism: F⁻ ions form O–H···F hydrogen bonds with MgO surface hydroxyl groups, while some F⁻ ions may undergo ion exchange with Mg²⁺ or form surface complexes, leading to effective fluoride ion immobilization.
In summary, the developed active MgO material offers advantages including simple preparation, high adsorption efficiency, and broad pH applicability, presenting a promising adsorbent option for advanced treatment of drinking water and fluoride-containing wastewater. Future research should focus on the material's recyclability, adsorption selectivity under complex real-water matrices, and the development of compact water purification devices based on this material.
Reference:https://pubs.acs.org/doi/10.1021/acsestwater.4c00403
RELATED NEWS
CATEGORIES
LATEST NEWS
CONTACT US
Name: Joy
Mobile:WhatsApp +86-15615531918
Tel:+86-15615531918
Whatsapp:+86 15615531918
Email:joy@onlyzone.cn
Add:Building 25-1, 333 Sanying Road, Zibo City, Shandong Province, China